Our Motivation
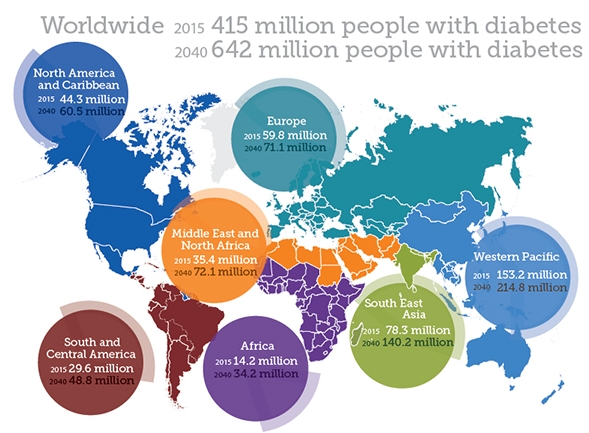
The number of people with diabetes is approaching 500 million. It is a disease that can be debilitating to patients and their families. Diabetes is characterized by high blood sugar levels as a result of insufficient insulin production from cells in the pancreas, called beta-cells. As a result, many with diabetes must take frequent daily injections of insulin and regularly measure blood glucose levels in order to attempt to minimize large glucose fluctuations. With this comes worry about the ongoing damage to the body that high blood glucose causes, but also the fear of low blood sugar when too much insulin is administered, which can cause coma, and if untreated, death. Parents treating young children with diabetes struggle with these facts. Joe Callander produced a short video for The New York Times depicting the hardships of life with diabetes, called ‘Midnight Three & Six’. Better options are desperately needed, and just like the Nobel Prize winning discovery of insulin by Canadians in 1921, they will come from research.
source: International Diabetes Federation, 2015. http://www.diabetesatlas.org
Our Approaches
The Kieffer lab has the capacity to address questions from molecular to cellular to whole organism, using model cell lines, differentiated stem cells, zebrafish and genetically engineered rodents. We utilize tissue-specific knockdown or reintroduction of genes, cell transplant and surgical manipulations to address the role of hormone actions in a site-specific manner. We assess the effects of environment on metabolic function with dietary manipulations, from neonates to adults. We have advanced equipment for high-throughput analyses of cellular function and pathways, for whole animal imaging, and for metabolic phenotyping. Through strong networks of collaborators including basic scientists and clinicians, we have assembled and led multidisciplinary teams and effectively engaged researchers across Canada and around the world to support our research enterprise. We actively collaborate with industry, including large pharmaceutical companies, as we strive to translate our findings to the clinical setting.
Our Projects
We seek to improve life for people affected by diabetes, by contributing to the development of new therapies, and ultimately a cure. We are pursuing many different approaches in the laboratory that all have potential to yield significant impact.
Physiological Insulin Replacement
We believe the best therapies for diabetes will come from approaches that re-establish automatic release of insulin within the body. Clinical studies involving transplant of pancreatic islets validate the effectiveness of this approach. Islets are small clusters of hormone producing cells, including the insulin producing beta-cells, and constitute only a few percent of the pancreas tissue. A few labs in Canada, including the Ike Barber Human Islet Transplant Laboratory here in Vancouver, can produce relatively pure preparations of islets isolated from the pancreas of recently deceased organ donors, and then transplant these into patients with diabetes. Since the amount of insulin producing tissue is small, only a few teaspoons, the transplant is relatively quick and simple (an infusion), and the patient can return home the same day. The results can be truly remarkable; with normal regulation of blood glucose levels achieved, patients can often stop taking insulin injections.
Continuous blood glucose monitoring from a patient with type 1 diabetes before (left) and 9 months after islet transplantation (right), demonstrating the efficacy of a cell therapy approach. EACH LINE REPRESENTS A DIFFERENT DAY. Healthy blood glucose levels are WITHIN THE GRAY SHADED AREA. Reproduced from: LATRES E, FINAN DA, GREENSTEIN JL, KOWALSKI A, KIEFFER TJ (2019) CELL METABOLISM 29:545-563.
Despite the clear success with islet transplant, the widespread adoption of this procedure is severely limited by: 1) the challenging islet isolation procedure; 2) the reliance upon limited organ donations; 3) the need for recipients to take chronic immunosuppression to prevent rejection of the transplanted cells. Our research seeks to address these limitations.
Stem Cells
Stem cells are very special cells that have the ability to multiply, and also to become all cell types of the human body. Stem cells can now be made from a patient’s own skin cells (induced pluripotent stem cells), thanks to methods developed by Shinya Yamanaka at Kyoto University, work that earned him a Nobel Prize in 2012. It is possible to grow large quantities of stem cells in the laboratory, and then to coax them into insulin producing cells. Research, including that of our own, indicates that these cells can effectively reverse diabetes after implant. Therefore, stem cells may be capable of providing a virtually unlimited supply of cells to treat millions of patients with diabetes. The California company ViaCyte, Inc. is now testing this approach in patients with type 1 diabetes, and we hope to initiate trials of this extremely promising therapy in Vancouver.
Encapsulation is a method to put a protective layer around the cells prior to implant, in order to prevent immune cells from attacking the new cells, while still allowing glucose and other nutrients to reach the cells, and insulin released from the encapsulated cells to enter the bloodstream. We have already conducted several successful experiments with macroencapsulation devices that are designed for transplant under the skin, and this approach is being tested in patients by ViaCyte. If successful, this may permit the implant of insulin producing cells derived from stem cells into patients with diabetes, without the need for chronic immunosuppression.
Powerful new methods in genetic engineering, such as CRISPR, now enable us to readily edit the genome of our cultured stem cells. In some patients diabetes is caused by a specific mutation in a gene important for the development and/or function of insulin producing beta-cells. Genetic engineering may enable us to correct such genes in patient derived induced pluripotent stem cells, which we can then use to generate functional beta-cells to transplant back into the patient in the hopes of curing their diabetes. We can also use genomic editing to disrupt the function of specific genes in stem cells to subsequently probe their function once the cells are differentiated to beta-cells. In this manner, we can use human stem cells as a very powerful model to better understand how diabetes develops.
Our ability to manufacture large quantities of human insulin producing cells also enables us to hunt for new drugs to treat diabetes by using high content screening. Using our robotics and high content, high throughput imagers, we can conduct thousands of experiments at a time, investigating the effects of small molecules on the survival and function of the insulin producing beta-cells. This research could identify novel approaches to treat diabetes.
Allard Cell Therapy Lab
The Allard Cell Therapy Lab was established in space within the UBC Life Sciences Institute directly adjacent to Dr. Kieffer’s lab, with funding generously donated by Charles R. Allard. It includes a tissue culture room for growth, differentiation, and cryopreservation of pluripotent stem cells. The primary goal of the Allard Cell Therapy Lab is to facilitate the development of clinically relevant procedures to cultivate cells that can be used to treat diabetes. The facility also produces cells that can be employed for research purposes, such as gene engineering studies, probing islet development pathways, and drug screening for new therapies.
Gene Therapy
As an alternative to cell transplantation, it may be possible to genetically engineer surrogate beta-cells by coaxing different cells in the body to take over insulin production. However, a key criteria for insulin replacement is that it must be produced in a meal dependent manner. We are exploring the feasibility of using gut K-cells for this purpose. These cells are located within the lining of the intestine and produce the hormone glucose-dependent insulinotropic polypeptide (GIP), a hormone that was discovered by Drs. John Brown and Raymond Pederson at UBC in the early 1970’s. Like beta-cells, the K-cells have the ability to sense glucose and rapidly release a hormone into the bloodstream. Thus the circulating profiles of GIP and insulin are very similar. Remarkably, as we reported in Science, K-cells engineered to produce insulin can entirely replace insulin production by beta-cells. Moreover, we found that insulin producing K-cells escape the autoimmune attack that destroys pancreatic beta-cells. In addition, insulin produced in the intestine appears to dampen the immune attack on beta-cells, raising the possibility that this approach could be used to prevent diabetes. Dr. Kieffer co-founded enGene, Inc., a biotech company that is striving to develop a gene pill, or oral formulation, to facilitate the clinical translation of this approach for the regulated delivery of insulin and other peptides that may improve glucose homeostasis and lower body weight.
Stem cell differentiation for cell therapy and disease modelling. Induced pluripotent stem (iPS) cells can be derived from somatic cells, such as skin cells, of healthy individuals or patients with a disease that would benefit from cell therapy, such as type 1 diabetes. Following induction of pluripotency, stem cell differentiation protocols can program iPS cells into the desired target cell. Following stem cell differentiation, a patient in need of cell therapy may receive a cell transplant originating either from their own cells or from a healthy individual. Alternatively, where a patient’s own cells contain a disease-inducing gene mutation, gene editing with the CRISPR/Cas9 system can correct the mutation, generating normal cells that could then be transplanted back into the same patient. Gene editing of normal cells can also be employed to probe the function of individual genes in cell function and disease etiology.
Beta-Cell Regeneration
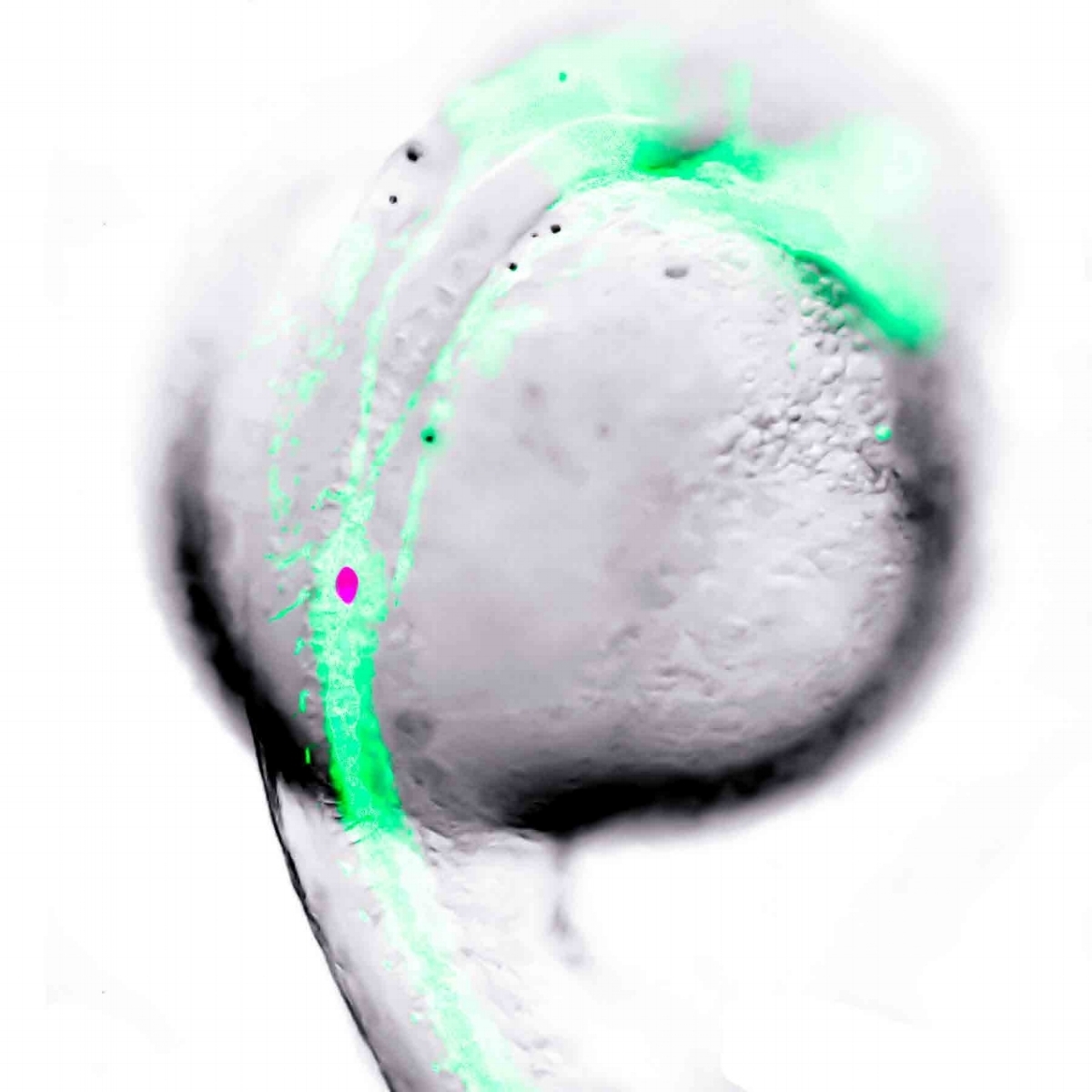
Perhaps an ideal way to cure diabetes will be to regenerate pancreatic beta-cells in patients with diabetes. Does the pancreas contain precursor cells that can be activated to form more beta-cells? Our studies with Dr. van der Kooy’s lab suggest there may be, although this remains controversial. We are studying beta-cells in zebrafish, as they have the remarkable capacity to rapidly regenerate beta-cells when needed. Moreover, beta-cell formation can be watched directly in the developing embryos, which are see-through. We have strains of zebrafish in which we can selectively destroy beta-cells to study the regenerative process, facilitated by the marking of beta-cells with fluorescent proteins that can be visualized under the microscope. Moreover, we have the ability to conduct these studies using our robotics and high content, high throughput imagers, such that we can screen thousands of compounds for small molecules that promote beta-cell regeneration. Zebrafish embryos are also highly amenable to genetic engineering to probe gene function and also generate novel lines of fish to facilitate new research. For example, we have generated zebrafish in which the beta-cells emit light when they are activated to release insulin. In this manner, we can screen the fish for small molecules that stimulate insulin secretion as a method to look for novel compounds that could be effective therapies for diabetes.
beta-cell development in the zebrafish. one day old transgenic zebrafish embryo with developing vasculature and beta-cells labelled with green and red fluorescent protein, respectively.
Hormones
Hormones are powerful regulators of metabolism and we are very interested in elucidating how hormones coordinate the precise regulation of glucose homeostasis. We are particularly interested in the pancreatic islet cells and hormone producing cells found in the intestine, including how the cells develop and how they function.
It has widely been assumed that insulin is the only hormone that can be used to control glycemia in diabetes, but it is now emerging that other hormones also have potent effects on blood glucose levels and these actions may be harnessed therapeutically. For example, during his PhD work at UBC with Dr. Raymond Pederson and Christopher McIntosh, Dr. Kieffer discovered that the insulinotropic actions of the gut hormones GIP and GLP-1 are limited by the enzyme DPP4. As a result of this finding, DDP4 inhibitors and DPP4 resistant GLP-1 analogs have been developed into new products to boost insulin levels in patients with type 2 diabetes. These drugs are now taken by millions of patients around the world. We have developed novel bioassays that report activity of GIP, GLP-1 and glucagon and are using these to look for small molecule modulators of these receptors that may be taken orally to treat diabetes and obesity.
Another hormone with potent glucose-lowering actions is leptin. Originally isolated from fat and found to act in the brain to induce satiety and increase energy expenditure, leptin has clinical utility for promoting weight loss, although leptin resistance limits its effectiveness in most obese patients. The actions of leptin to regulate glucose homeostasis are even more potent; even in the setting of type 1 diabetes, leptin can dramatically lower blood glucose levels. We are using multiple complementary approaches to elucidate how leptin has these actions in the hopes of identifying new therapeutic targets. We are also investigating how leptin resistance develops in the context of obesity in the hopes of identifying novel approaches to promote weight loss.
Glucagon is a powerful counter-regulatory hormone that opposes the action of insulin; when glucose levels are too low, glucagon produced from the neighbouring pancreatic islet alpha-cells acts to raise blood glucose. Interestingly, inappropriately high glucagon levels contribute to excess levels of glucose in the blood, such that glucagon antagonists are being investigated to treat diabetes. We are further probing the actions of glucagon and seeking novel ways to reduce its action.
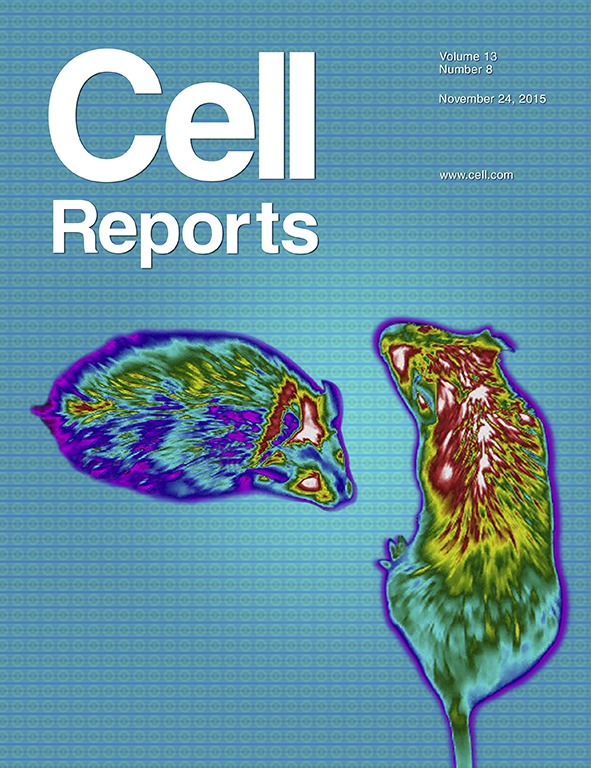
On the cover: Kwon et al. use infrared thermal imaging to measure body surface temperature near interscapular BAT during a glucose meal in vehicle and FGF21-treated mice. Images depict body surface heat distribution of mice during such meal. The study reveals that there is a decrease in BAT temperature during glucose excursions and that FGF21 improves glucose clearance while preventing the fall in BAT temperature.